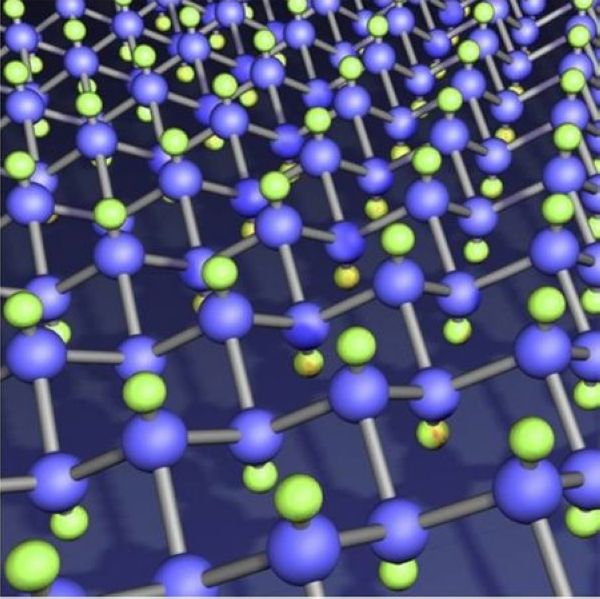
As a new type of solid lubricant, Fluorinated Graphite Lubricant has better lubricity than graphite and aluminum disulfide, especially in harsh atmosphere, high speed, high pressure and high temperature conditions, and it has better effect, and does not corrode metals and other materials. The reason why Fluorinated Graphite Lubricant has such excellent lubricating properties is mainly because fluorine atoms enter between the graphite layers and form covalent bonds with the electrons, resulting in a significant reduction in the bond energy between the graphite layers, only 2Kcal/mol, which is far greater than that of the raw graphite. The interlayer energy of 9Kcal/mol is as low as 9Kcal/mol, which is the fundamental reason for the excellent lubricating properties of Fluorinated Graphite Lubricant. In addition, because the upper and lower surfaces of the graphite hexagonal network plane layer are densely bound with fluorine atoms, and the fluorine atoms between the layers have repulsive forces with each other, which can offset the pressure from the outside, so the Fluorinated Graphite Lubricant can fully express Excellent lubricating properties.
At a temperature of 320 °C, the friction coefficient of graphite is 0.53, while that of Fluorinated Graphite Lubricant is only 0.10.
As the fluorine content increases, graphite changes from gray-black to snow-white; the higher the degree of fluorination, the better the lubricating properties of Fluorinated Graphite Lubricant.
Fluorinated Graphite Lubricant
Introduction

As a new type of solid lubricant, Fluorinated Graphite Lubricant has better lubricity than graphite and aluminum disulfide, especially in harsh atmosphere, high speed, high pressure and high temperature conditions, and it has better effect, and does not corrode metals and other materials. The reason why Fluorinated Graphite Lubricant has such excellent lubricating properties is mainly because fluorine atoms enter between the graphite layers and form covalent bonds with the electrons, resulting in a significant reduction in the bond energy between the graphite layers, only 2Kcal/mol, which is far greater than that of the raw graphite. The interlayer energy of 9Kcal/mol is as low as 9Kcal/mol, which is the fundamental reason for the excellent lubricating properties of Fluorinated Graphite Lubricant. In addition, because the upper and lower surfaces of the graphite hexagonal network plane layer are densely bound with fluorine atoms, and the fluorine atoms between the layers have repulsive forces with each other, which can offset the pressure from the outside, so the Fluorinated Graphite Lubricant can fully express Excellent lubricating properties.
At a temperature of 320 °C, the friction coefficient of graphite is 0.53, while that of Fluorinated Graphite Lubricant is only 0.10.
1. Product Name
Fluorinated Graphite Lubricant, Fluorographite polymer
2. Composition /Information on Ingredients
Polyfluorocarbon and fluorocarbon .
Molecular formula: (CFx)n, 0<X<1.25
3. Product Categories
According to the degree of fluorination, Fluorinated Graphite Lubricant can be divided into:
The low fluoride graphite: CF (0-049)
The medium fluoride graphite: CF (0.5~0.99)
The high fluoride graphite: CF (1.0~1.25)
As the fluorine content increases, graphite changes from gray-black to snow-white; the higher the degree of fluorination, the better the lubricating properties of Fluorinated Graphite Lubricant.
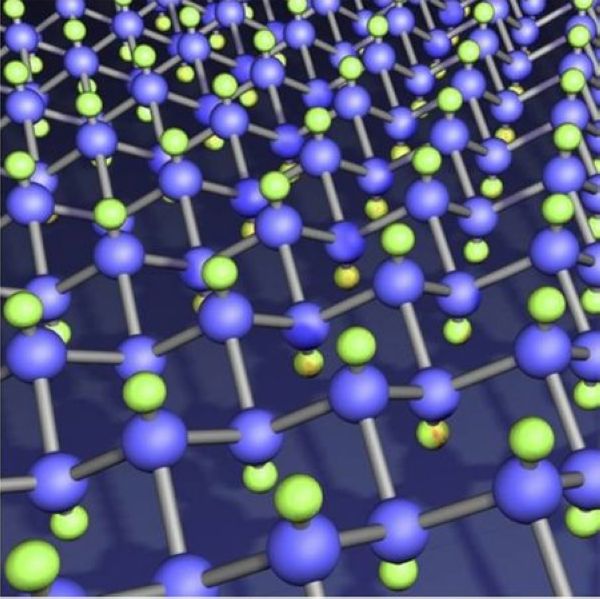
FIG.1 Molecular structure of Fluorinated Graphite Lubricant.

FIG.2 Graphite Fluoride Lubricant for Aerospace Lubricants.
On January 30, 2023, molybdenum price rose by more than 20% in a single day, breaking a new 17-year high. Molybdenum is known as the "war metal", "steel warrior". Molybdenum is valued by more and more countries. China is the world's richest country in molybdenum resources, with reserves of 4.3 million tons, followed by the United States (2.7 million tons) and Chile (1.8 million tons), which acco...more